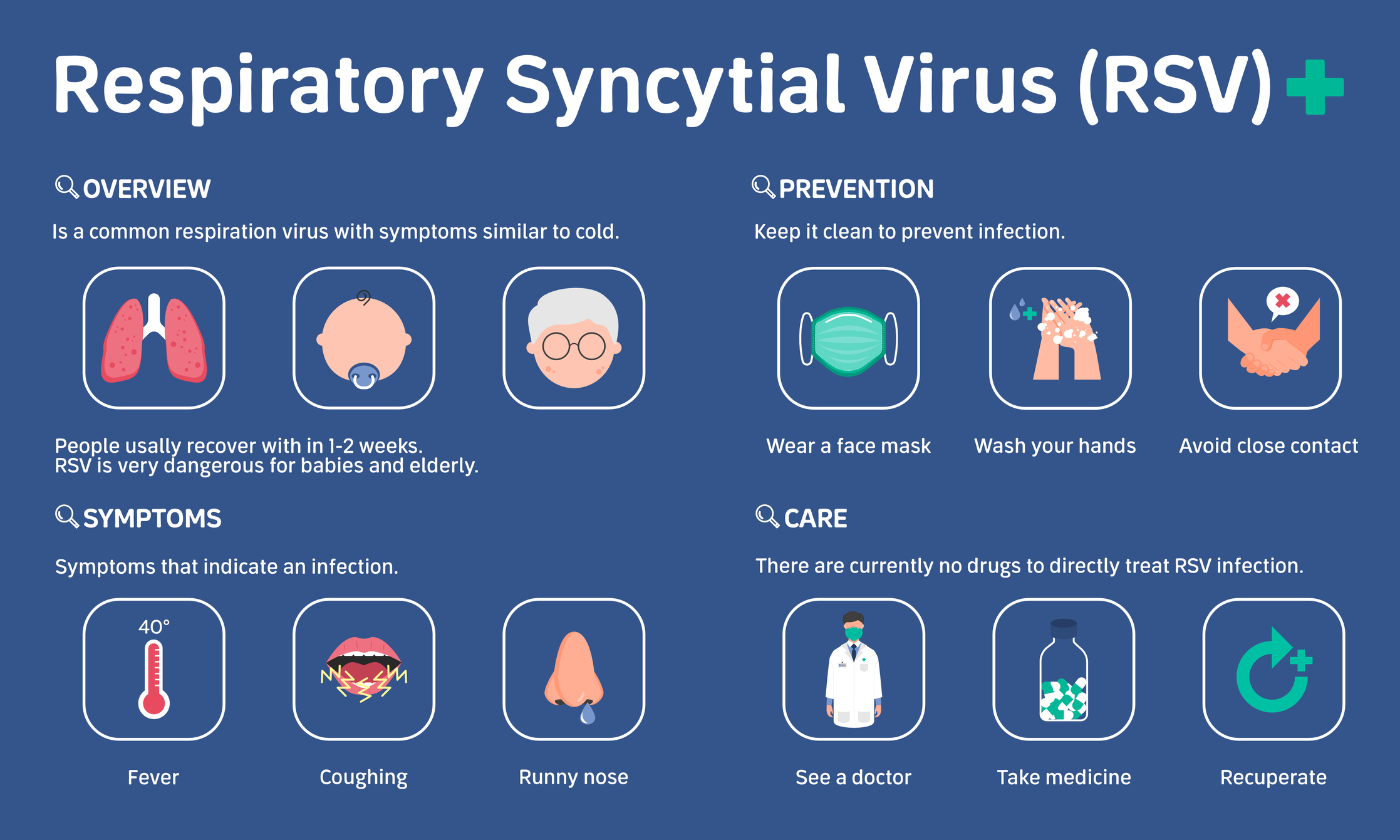
A subtle shift occurs in August – the days are just a little shorter and evenings become cooler, triggering thoughts of cozy sweaters, pumpkin spice, and autumn leaves. Fall is also a time for older adults to start planning for their annual flu and pneumonia vaccine or COVID booster. This year, adults over the age of 60 may be able to get their shot for the first respiratory syncytial virus or RSV vaccine which just received approval from Health Canada.
According to a recent CBC Health News report, the RSV vaccine may only be available in Canada in limited quantities this fall. RSV is a common, highly contagious virus that in vulnerable populations, like young children or older adults, can cause inflammation in the airways. The United States Food and Drug Administration also gave its approval for the vaccine in May.
Seniors who contract RSV are at increased risk for severe illness, hospitalization, and death. The vaccine has been found to be effective at significantly reducing the risk of lower respiratory tract disease by 82 percent and is 94 percent effective at preventing illness in seniors with underlying health conditions.
In addition to causing breathing problems among older adults, RSV infection can also lead to bacterial or viral pneumonia, or trigger other heart or lung complications that can be life-threatening. The RSV season aligns with the annual flu season running from late fall until spring.
Health Canada has stated the RSV vaccine will be administered with a single dose injection but is unclear whether the shot will be required each year or if older adults will be offered a booster. More guidance is expected in the new year. Read more about the RSV vaccine, recommendations for use, and any side effects by following this link to the Government of Canada website.




Add Your Voice
0 Comments
Join the Discussion