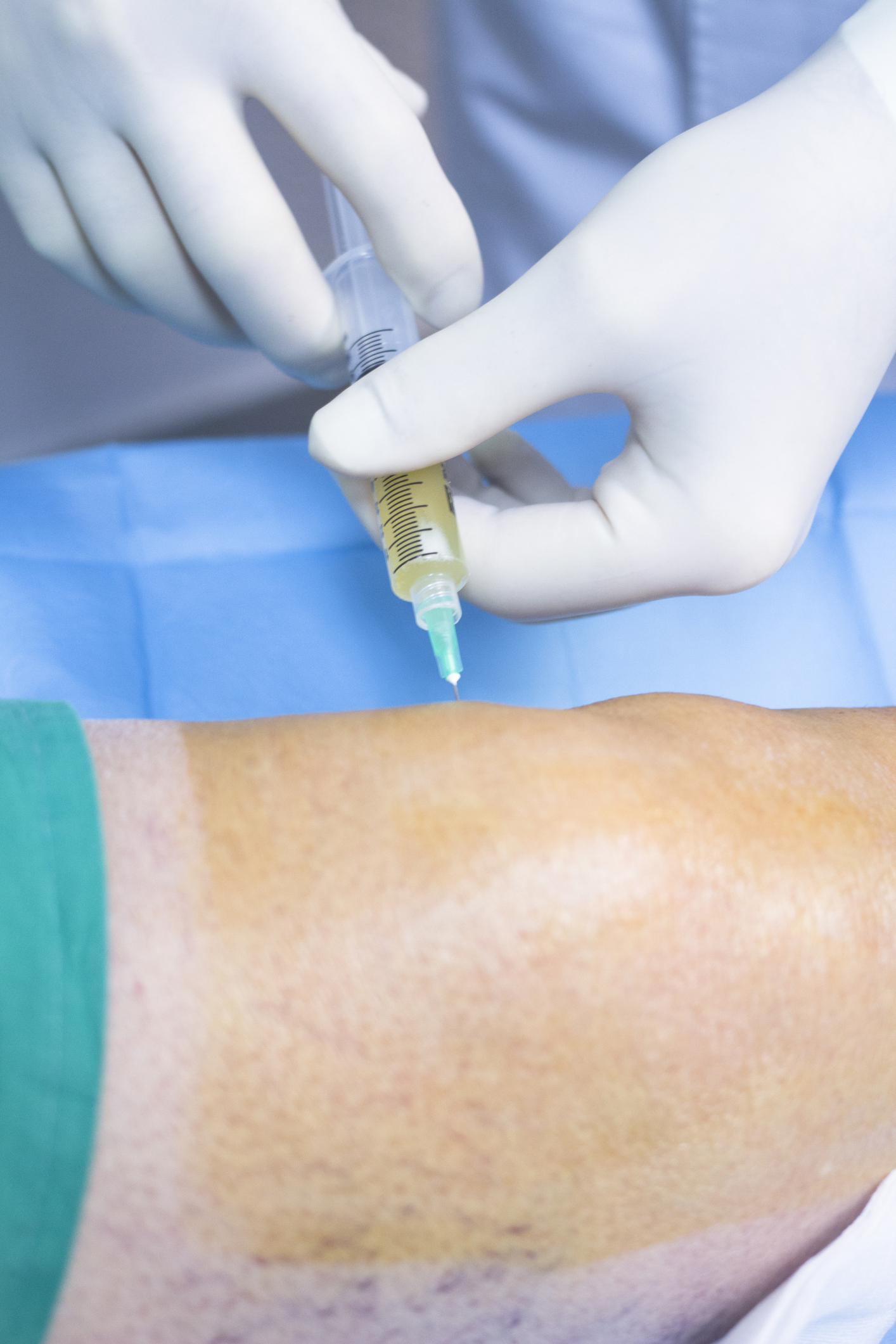
Aging baby boomers have been flocking to private clinics popping up in Canada and the United States offering stem cell treatments that promise to alleviate pain and and even repair damaged tissue associated with degenerative arthritis and age related conditions like bursitis and degeneration of cartilage and ligaments. Some even treat patients with Parkinson’s disease, COPD(Chronic Obstructive Pulmonary Disease), heart disease or ALS (amyotrophic lateral sclerosis) without any scientifically tested benefit.
Unproven and costly, Health Canada is investigating these clinics to make sure they comply with the Food and Drug Act but because the material injected into clients has been “minimally manipulated,” the clinics may fall outside the government’s jurisdiction. According to a recent CBC report, in most cases, the stem cells are removed from a patient’s own bone or fat tissue, spun in a centrifuge and then injected into the site of pain or injury. The theory is that the cellular material will speed healing and relieve pain.
However, it is important to understand that this is not a pure stem cell procedure which are, as of yet, only available through clinical trials where cells are removed and expanded in a lab and then tested for safety before being injected into a knee or hip. In private clinics, the injected material is not a pure product and may include concentrated bone marrow or broken down fat tissue.
In the U.S., the FDA is also cracking down on clinics that offer untested stem cell therapy. Some of the clinics may pose a danger to patients and research has found that regulatory agencies must better oversee this commercial activity. According to a FDA press release from August 28, 2017, “Stem cell clinics that mislead vulnerable patients into believing they are being given safe, effective treatments that are in full compliance with the law are dangerously exploiting consumers and putting their health at risk.” The FDA also states that while the goal is to provide a efficient framework for promising technologies, regulations are in place to protect patients who may be at risk from infection from unsanitary conditions or by expensive treatments which may provide little or no benefit.
To learn more about the regulation of private stem cell clinics in a study published recently in the Journal Cell Stem Cell follow this link.





Add Your Voice
0 Comments
Join the Discussion