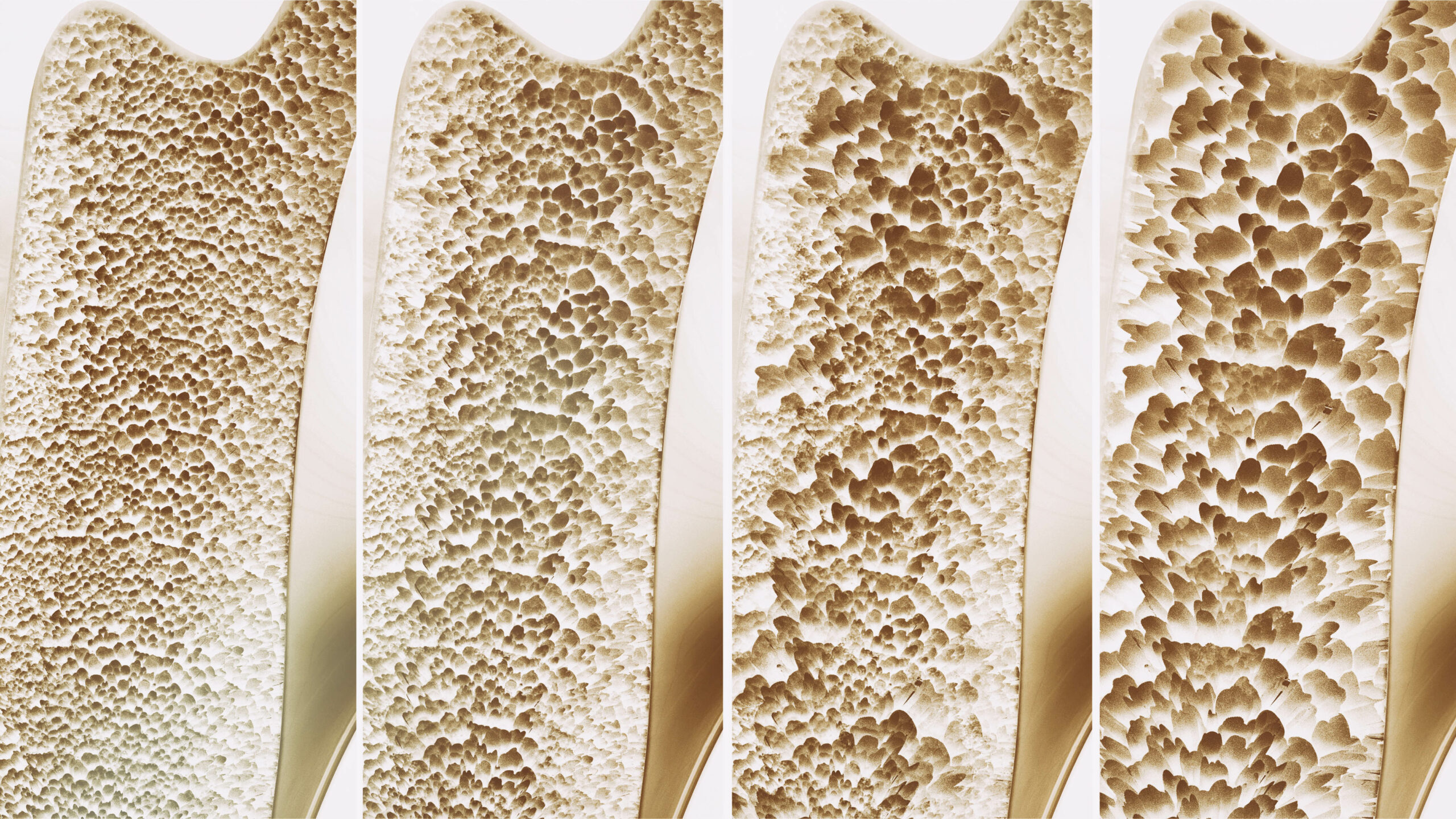
Loss of bone density puts older adults at increased risk for fractures, often of the hip or spine, that frequently lead to a loss of function and independence. In the United States alone, an estimated 10 million people have osteoporosis and although there are drugs to help preserve bone density, until recently there has been no approved treatment to help build bone.
According to a recent New York Times report, the U.S. Food and Drug Administration approved a new drug Tuesday for the treatment of osteoporosis, a condition which causes more bone to be broken down by the body than built up. The newly approved drug, romosozumab-aqqg (brand name Evenity) is approved for postmenopausal women with a high risk for fracture. In trials of the drug, patients experienced a reduction in fractures as well as a significant increase in bone density.
However, Evenity, which is administered through a monthly injection also carries a small increase in the risk for heart attacks, strokes and sudden death. Patients who have had a heart attack or stroke in the past year should not take the drug and those who experience a heart attack or stroke while on the medication should stop taking Evenity. Once patients increase their bone density, researchers suggest switching to an older drug that will help maintain the new bone growth.
Side effective of Evenity also may include joint pain, headaches and irritation at the injection site. The FDA approval of this drug to treat osteoporosis, which affects an average of 1 in 3 women and 1 in 5 men, is the first new therapy in nearly 20 years. The research leading up to the discovery of this drug is based on the study of a genetic mutation among Afrikaners in South Africa, sclerosteosis, which causes bones to thicken. The gene blocks a specific protein, sclerotin, which prevents bone growth. This research was instrumental in the development of the drug romosozumab-aqqg which stimulates this gene, helping to rebuild weakened bones.
The patient cost of Evenity has not yet been released but it is expected to be unveiled next week. The drug has already received approval in Japan is under review in Europe. Analysts predict the drug’s earning potential to reach an estimated $86 million in 2020 sales.
Learn more about Evenity and the treatment of osteoporosis by following this link to the drug’s developer, the biotechnology company Amgen’s website.





Add Your Voice
0 Comments
Join the Discussion